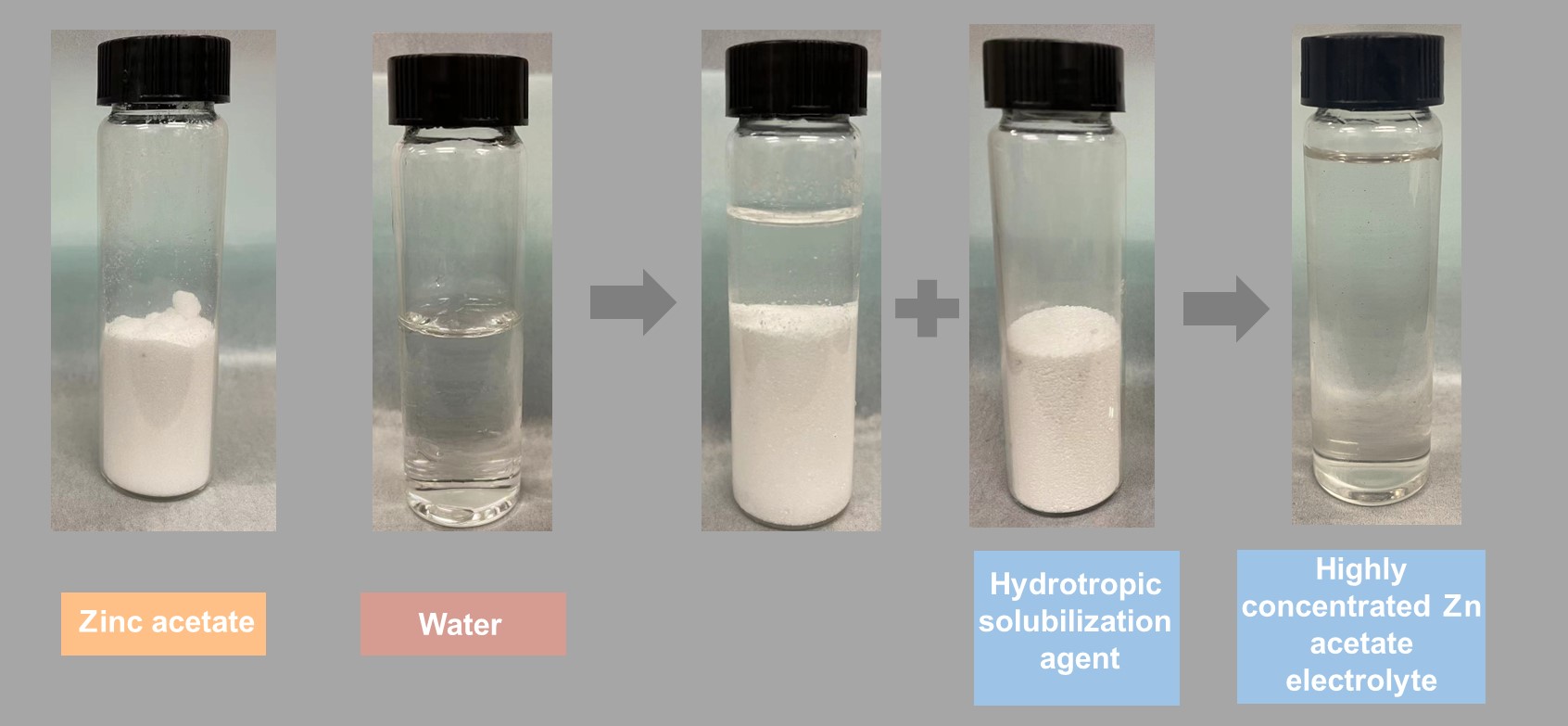
A research team led by Professor Lu Yi-chun in the Department of Mechanical and Automation Engineering at The Chinese University of Hong Kong (CUHK) has taken a critical step forward in creating a high-performance, eco-friendly electrolyte for aqueous zinc batteries. The team applied a strategy from pharmaceutical science to increase drug solubility, solving the problems of aqueous zinc batteries’ short life span by enabling highly concentrated zinc acetate electrolytes, a halogen-free zinc salt. The breakthrough was recently published in the world-leading scientific journal Nature Sustainability, a sister journal of Nature. Making aqueous zinc batteries more powerful and sustainable Lithium ion (Li-ion) batteries are widely used in electronic devices and gadgets such as mobile phones and laptops. Owing to their stable energy output and satisfactory lifespan, they have become the heart of these portable electronics, as well as electric vehicles and large-scale solar and wind farms. However, Li-ion batteries still heavily rely on toxic, flammable organic electrolytes, which come with serious safety concerns. In contrast, aqueous zinc batteries are non-flammable and, thanks to their water-based electrolyte, do not pose any significant risk of explosion. However, the zinc in the batteries suffers greatly from side reactions and has a short life span. Repetitive zinc metal deposition and dissolution while the batteries are operating leads to the growth of needle-like zinc structures, which can short-circuit the batteries. Existing approaches to mitigating the needle-like zinc often involve the use of a large amount of halogen-containing salts, which raises issues of environmental sustainability. Dietary supplement ingredient helps stabilise zinc batteries The hydrotropic solubilisation effect is a common phenomenon used in the pharmaceutical field to increase the water solubility of drugs. Poorly water-soluble drugs can be easily dissolved in water with the help of hydrotropic solubilisation agents, which interact strongly with both water and the drug molecules. Inspired by this phenomenon, Professor Lu’s team successfully developed several hydrotropic solubilisation agents to boost the solubility of the poorly water-soluble halogen-free zinc acetate by up to 14 times. One of the effective hydrotropic solubilisation agents demonstrated in the study was urea, which is non-toxic, eco-friendly and often used in topical dermatological products to promote hydration of the skin. Using the highly concentrated zinc acetate enabled by urea, a full zinc battery demonstrated a satisfactory life span of more than 4,000 cycles even with fast charging (in 6 minutes). The common side reactions that aqueous zinc batteries suffer from, such as hydrogen gas evolution, are virtually eliminated. The electrolyte allows high-performance zinc batteries to operate in a much more environmentally sustainable way. Professor Lu said, “Our work provides a rational, universal approach to breaking the solubility limit of cost-effective, eco-friendly salts for sustainable, high-performance battery applications. It will unlock the potential of many eco-friendly materials to be used for clean energy applications.” Reference: Dejian Dong, Tairan Wang, Yue Sun, Jun Fan, Yi-Chun Lu*. Hydrotropic solubilisation of zinc acetates for sustainable aqueous battery electrolytes. Nature Sustainability (2022). |
|